That’s why millions of Americans take blood-thinning medications, such as warfarin, that make it harder for their blood to clot.
However, patients need to be tested frequently to make sure their blood is in the correct range that clots too easily could still lead to a stroke or a heart attack while blood that doesn’t clot can lead to extended bleeding after an injury.
To be tested, patients either have to go to a clinical laboratory or use a costly at-home testing system. The newly developed blood clotting test overcomes these difficulties.
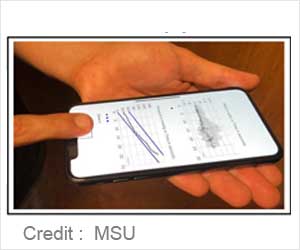
Using a new testing method, a person adds a drop of blood to the cup, which contains a small copper particle and a chemical that starts the blood-clotting process.
Then the phone’s vibration motor shakes the cup while the camera monitors the movement of the particle, which slows down and then stops moving as the clot forms.
Researchers showed that this method falls within the accuracy range of the standard instruments of the field. These findings are published in Nature Communications.
“Back in the day, doctors used to manually rock tubes of blood back and forth to monitor how long it took a clot to form. This, however, requires a lot of blood, making it infeasible to use in home settings,” said senior author Shyam Gollakota, UW professor in the Paul G. Allen School of Computer Science & Engineering.
Doctors can rank blood-clotting ability using two numbers: the time it takes for the clot to form, what’s known as the “prothrombin time” or PT.
A ratio calculated from the PT that allows doctors to more easily compare results between different tests or laboratories called the “international normalized ratio” or INR.
Patients who can monitor their PT/INR levels from home would only need to go to see a clinician if the test suggested they were outside of that desirable range.
To calculate PT and INR, the phone collects two-time stamps: first when the user inserts the blood and second when the particle stops moving.
Researchers tested this method on three different types of blood samples. As a proof of concept, the team started with plasma, a component of blood that is transparent and therefore easier to test.
They tested plasma from 140 anonymized patients at the University of Washington Medical Center. The team also examined plasma from 79 patients with known blood-clotting issues. For both these conditions, the test had results that were similar to commercially available tests.
This device is still in a proof-of-concept stage. The researchers have publicly released the code and are exploring commercialization opportunities as well as further testing.
The next step is to work with patients to test this system at home. The researchers also want to see how the system fares in more resource-limited areas and countries.
Source: Medindia