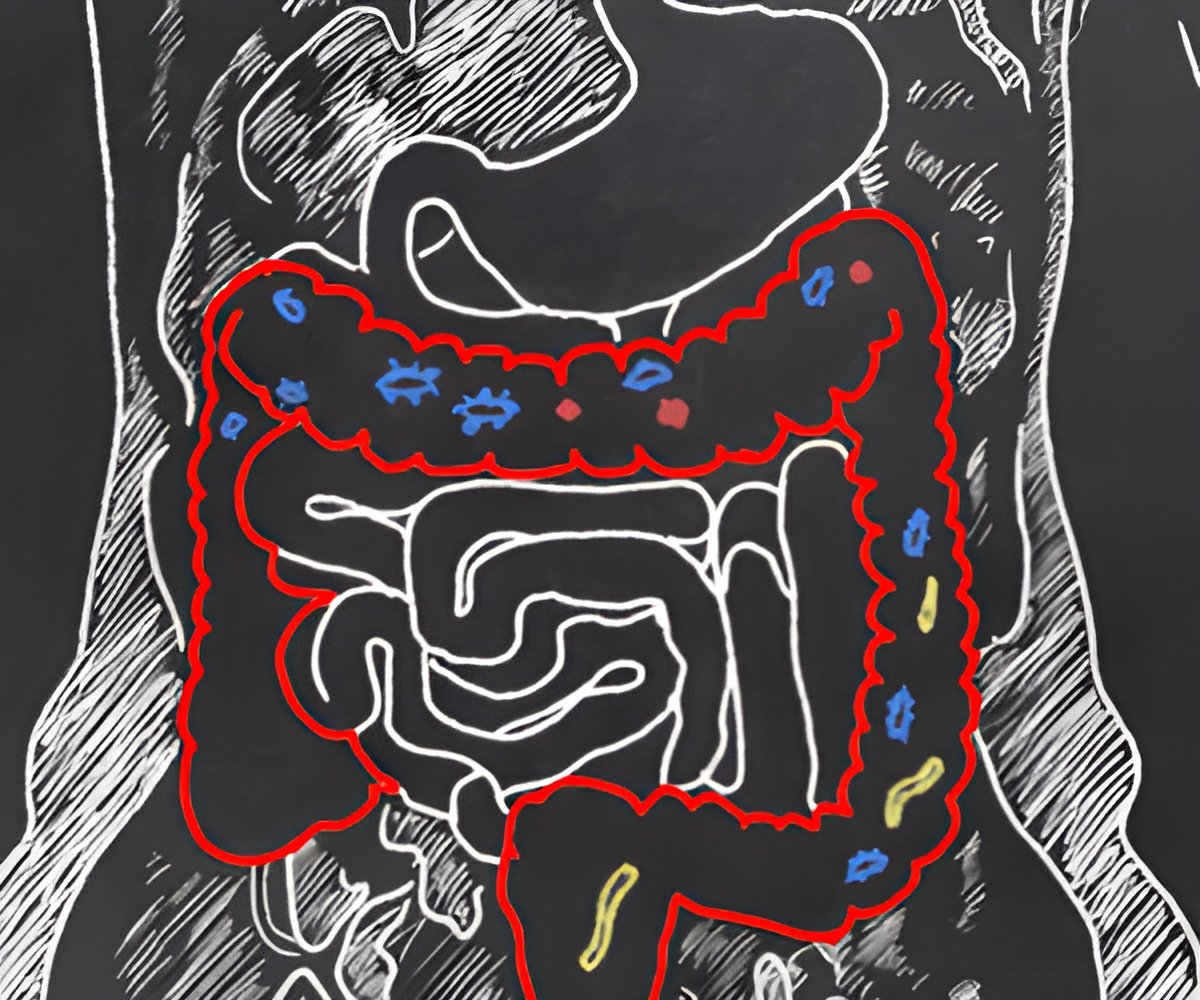
New research indicates that early life exposure to harmful chemicals known as “forever chemicals” can permanently damage the gut microbiome, leading to metabolic diseases like obesity and type 2 diabetes later in life. The study, conducted on mice, suggests these chemicals may be contributing to the rising rates of these disorders in adults.
The researchers focused specifically on 2,3,7,8-tetrachlorodibenzofuran (TCDF), a widespread persistent organic pollutant (POP) that is a byproduct of waste incineration, metal production, and fossil-fuel and wood combustion. TCDF accumulates in the food chain, and humans are primarily exposed through consumption of high-fat foods, such as meat, dairy products and some fish. Babies can be exposed through consumption of breast milk.
Chemical Exposure and Lasting Gut Microbiome Damage
“POPs are pervasive in the environment and nearly every living organism has been exposed,” said Andrew Patterson, John T. and Paige S. Smith Professor of Molecular Toxicology and of Biochemistry and Molecular Biology, Penn State. “The negative health effects of these chemicals are well documented and include birth defects and cancer. Our study is the first to suggest that early-life exposure to a certain POP, called TCDF, also disrupts the gut microbiome and is associated with metabolic disorders later in life.”
The team examined the effects of TCDF in two groups of mice — a test group, or those treated with TCDF and a control group, or those receiving no treatment. The team fed four-week-old mice from pills containing either 0.46 micrograms (µg) of TCDF or a control pill that did not contain any TCDF for five days. While 0.46 µg is higher than what is typically found in the diets of humans, it is not high enough to cause toxic illness.
“In our study we used a dose that is relatively high compared to typical human exposures; however, we can use this information to identify new toxicity high points, including in the gut microbiome, and begin to extrapolate what might happen at even lower doses. Of course, we also must consider how complex mixtures of these POPs interact with us and our microbial partners because a single exposure does not perfectly mimic real life scenarios.”
Next, the researchers examined the animals’ gut microbiomes, along with several indicators of the animals’ health, including body weight, glucose tolerance, and the amounts of triglycerides in their livers and mucus in their feces, among other markers of metabolic disease. They collected these data immediately following the five-day course of TCDF, as well as three months after the last dose. In humans, these time points are equivalent to an infant and a young adult.
To further explore the effects of TCDF on the gut microbiome, the scientists gave mice without microbiomes intestinal microbiome transplants from the mice with TCDF-disrupted microbiomes and measured their health outcomes. They found that the mice with the transplants developed metabolic disorders, indicating that the altered microbiome is the cause of the metabolic disease.
Advertisement
“These results suggest that early-life TCDF exposure may be causing the disturbances in gut microbiome function and health outcomes later in life, even well after the TCDF has been eliminated from the body,” Tian said.
She explained that the gut microbiome disturbances were marked by a decrease in certain bacterial species, including Akkermansia muciniphila, a bacterium that is also typically found in the human gut microbiome.
Advertisement
“This is important because Akkermansia is recognized as important for overall gut health, but now we know that it can be adversely affected by TCDF,” Tian said.
To investigate the importance of Akkermansia muciniphila in influencing health outcomes, the team experimented with administering the bacterium as a probiotic to TCDF-treated mice. The probiotic restored the microbiome to its normal state.
“Our findings suggest that these bacteria are influenced by toxic exposure and play an important role in mediating health outcomes,” Patterson said. “It may be possible that with more research we could one day restore a person’s microbiome to its optimal state through supplementation with pre- and probiotics.”
Source-Eurekalert