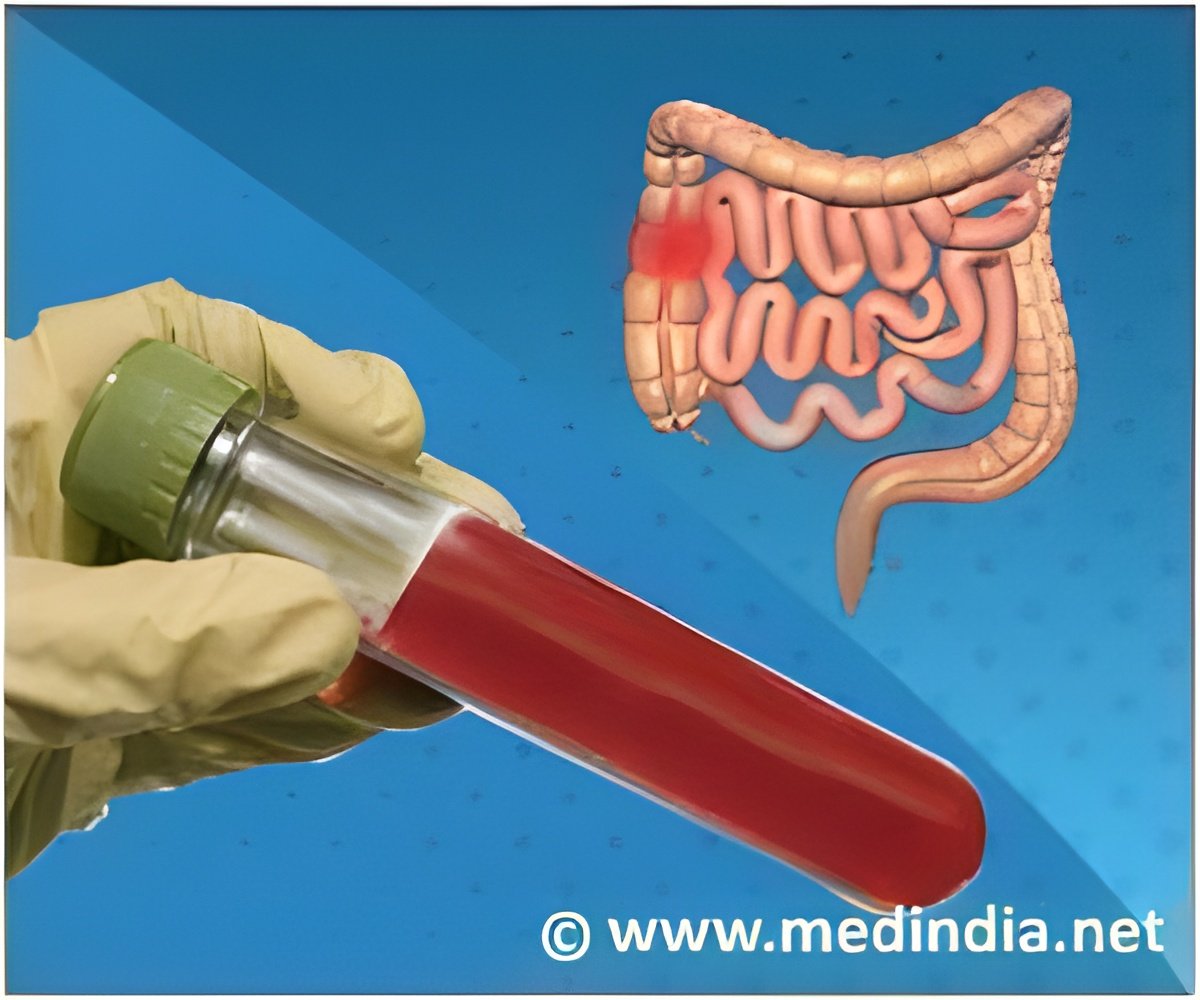
FDA-approved blood test for colorectal cancer is convenient but less accurate than a colonoscopy.

Shield, a newly approved cell-free DNA blood-based test for cancer detection may give false positive results. This has raised a concern about improving the technique.
Blood-based screening tests are a non-invasive procedure that can be used in the detection of cancer. Dr William Grady, MD, highlights the accuracy of Shield and other blood-based tests.
FDA-Approved Blood Test: Is It Accurate?
The U.S. Food and Drug Administration (FDA) approved this test based on the study published in the New England Journal of Medicine. They involved 7,861 patients and compared a cell-free DNA blood test with a colonoscopy for colorectal cancer screening.
The results obtained were a sensitivity of 13.2% for advanced precancerous lesions and a 65% sensitivity for detecting stage I cancer, with 10.1% false-positive results.
Another noninvasive stool test approved in 2014 showed better accuracy with sensitivity in detecting advanced precancerous lesions is 42.4% with DNA testing and 23.8% with fecal immunochemical testing (FIT). For stage 1 cancer, the sensitivity is approximately 88% with DNA testing and 64% with FIT. The false-positive rates are as high as 13.4% and 5.1%, respectively.
Why Colonoscopies are Important
This has created a debate among doctors about the accuracy of newly approved blood-based screening test. The questions arise about the certainty of a negative test, the ability to reassure patients, legal and ethical responsibilities if failed to analyze the precancerous or stage 1 cancer, counseling for false-positive results, and the frequency of test repetition.
Doctors argue that though it offers a noninvasive and versatile screening test, it is important to make people aware and reduce the fear of colonoscopies as it remains the most accurate and effective method for detecting and preventing colorectal cancer.
Advertisement
Source-Medindia