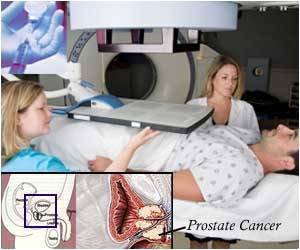
Throughout the past 40 years, randomized trials have been conducted on the impact of adding hormone therapy to prostate cancer treatments. While these trials individually show the benefit of hormone therapy, there are inconsistencies in the timing and duration of treatment recommendations.
“Our research team set out to conduct a first-of-its-kind, comprehensive analysis by collecting individual patient data from every randomized trial conducted around the world, and performed a meta-analysis of the impact of various treatment intensification strategies using hormone therapy with radiation therapy for localized prostate cancer,” said senior author Daniel E. Spratt, MD, Vincent K. Smith Chair in Radiation Oncology at UH Seidman Cancer Center, Professor in the Department of Radiation Oncology at Case Western Reserve School of Medicine, and Member of the Developmental Therapeutics Program at Case Comprehensive Cancer Center.
In this analysis, the team made three key discoveries:
1) Men with intermediate- and high-risk prostate cancer have an increased survival rate from the addition of hormone therapy to radiotherapy. This was seen in both younger and older men, and men treated with lower and higher doses of radiotherapy.
2) Survival rate in men with prostate cancer improves with the prolongation of adjuvant hormone therapy to radiotherapy. This benefit was seen in both younger and older men, in men treated with lower and higher doses of radiotherapy, and in men with both intermediate- and high-risk prostate cancer.
3) The prolongation of neoadjuvant hormone therapy before radiotherapy did not benefit men in any outcome measure.
Researchers showed that treating a group of approximately ten to 15 men with hormone therapy or extended adjuvant hormone therapy, for at least 18 months, prevented one man from developing metastatic disease ten years after treatment.
This is dependent on patient and tumor-specific factors but gives us a more precise estimate to work with when it comes to recommending treatment options.
The Meta-Analysis of Randomized Trials in Cancer of the Prostate (MARCAP) Consortium, is the first, comprehensive, international collaboration of randomized phase III clinical trial individual patient data.
This work from the MARCAP consortium will bring confidence in recommending various treatment intensification strategies, and allow providers to have more accurate, shared-decision-making conversations with patients about the benefits of using hormone therapy with radiotherapy for prostate cancer treatment.
In this MARCAP analysis, 12 randomized trials were included. The research team now has more than 20 trials, and that number is continuing to grow, from groups from around the world that have agreed to share data.
In the next steps for this research, this repository will be used to investigate additional clinically relevant questions regarding optimal dosing of radiotherapy, fractionation, use of pelvic nodal radiotherapy, and extending studies into the recurrent and advanced disease states.
Source: Medindia