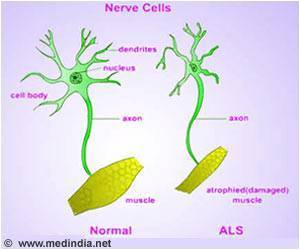
Most of the patients live for only about 2-4 years after the diagnosis as they succumb to motor degeneration and ultimately paralysis. The world-famous theoretical physicist –
However he survived for another 55 years, defying the odds.
‘Scientists uncover a significant role of proteins called optineurin (OPTN) in inhibiting the formation of ALS (amyotrophic lateral sclerosis) pathology-related protein aggregates.
’
Role of Proteins
The study team has demonstrated that a protein called optineurin (OPTN) is generally found to inhibit the formation of Ub-TDP-43 aggregates in stress-induced stress granules (SGs). But the OPTN mutants derived from the ALS patients induces Ub-TDP-43 aggregates in SGs.
In response to several stressors like heat shock and oxidation, the SGs are generated in the cytoplasm of cells. When the degree of stress diminishes, these SGs are subsequently eliminated.
The OPTN knockdown (OPTN-KD) cells increased the size of SGs. The team also established a direct link between OPTN-KD-induced increases in TIA1 protein and the formation of Ub-TDP-43-positive SGs in OPTN-KD cells.
Thus a delay in SG clearance and an accumulated Ub-TDP-43 in SGs after stress removal is characteristic of aberrant SGs. This induces toxicity to neurons, which manifests as characteristic neuron degeneration was seen in ALS patients.
“This discovery elucidates the process by which Ub-TDP-43 aggregates develop in the brains of ALS patients with optineurin or TIA1 mutations and explains how ubiquitinated proteins aggregate in neurodegenerative disorders such as ALS,” Prof. Masahiro Fujii of Niigata University Graduate School of Medical, who headed the study.
Source: Medindia