,” said Patricia Becerra, Ph.D., chief of NEI’s Section of Protein Structure and Function and senior author of the study.
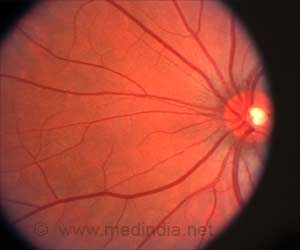
The retina is composed of layers of cells that function together to detect and process light signals, which the brain uses to generate vision. The retina’s light-sensing photoreceptors sit above the retinal pigment epithelium (RPE), a layer of support cells.
The RPE nourishes photoreceptors and recycles pieces of the photoreceptor cells called “outer segments,” which get used up and their tips shed each time photoreceptors detect light.
Advertisement
If the RPE cannot provide recycled components of older outer segment tips back to photoreceptors, these cells lose their ability to make new segments and eventually become unable to sense light.
And without nutrients supplied by the RPE, photoreceptors die. In people with AMD or certain types of retinal dystrophies, senescence (aging) or the death of RPE cells in the retina leads to vision loss.
Previous work has shown that PEDF protects retinal cells, preventing both damage to the cells and abnormal growth of blood vessels in the retina. RPE cells produce and secrete the PEDF protein. The protein then binds to its receptor, PEDF-R, which is also expressed by RPE cells.
Binding by PEDF stimulates PEDF-R to break down lipid molecules, key components of the cell membranes that enclose photoreceptor outer segments and other cellular compartments.
This breakdown step is a key part of the outer segment recycling process. And while researchers have known that PEDF levels drop in the retina during the aging process, it was not clear whether this loss of PEDF was causing, or merely correlated with, age-related changes in the retina.
What Is The Role Of ‘Youth’ Protein In Aging Eye?
To examine the retinal role of PEDF, researchers studied a mouse model that lacks the PEDF gene (Serpin1). The researchers examined the cellular structure of the retina in the mouse model, finding that the RPE cell nuclei were enlarged, which may indicate changes in how the cells’ DNA is packed.
The RPE cells also had turned on four genes associated with aging and cellular senescence, and levels of the PEDF receptor were significantly below normal.
Finally, unprocessed lipids and other photoreceptor outer segment components had accumulated in the RPE layer of the retina. Similar changes in gene expression and defects in RPE metabolism are found in the aging retina.
While at first glance, the retinas of these PEDF-negative mice appear normal, these new findings suggest that PEDF is playing a protective role that helps the retina weather trauma and aging-related wear and tear.
Researchers were wondering if the loss of PEDF was driven by aging, or was driving aging. This study, especially with the clear link to altered lipid metabolism and gene expression, indicates the loss of PEDF is a driver of aging-related changes in the retina.
Source: Medindia