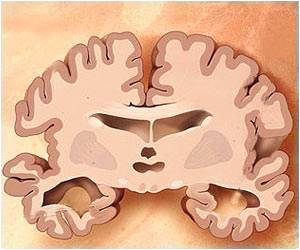
The healthy function of biological cells is maintained by tau protein. However pathological clumping of these tau protein aggregates blocks the firing of neurons, leading to a wide range of other neurodegenerative diseases known as tauopathies.
‘Tau accumulation and its toxicity in the Alzheimer’s brain can be triggered by a specific chemical feature of the protein such as disulfide bonds and exposure to an environment with elevated levels of reactive oxygen species (stress). Thus identifying these chemical targets that trigger tau accumulation may lead to the formulation of breakthrough treatments for Alzheimers disease and other tauopathies.’
Molecular Mechanism for Tau Toxicity
The study team found that alterations to amino acid residues in the protein known as cysteines in two different locations (C291 and C322) had a drastic effect on the amount and toxicity of tau.
Moreover, the disulfide bonds formed by these cysteine groups and exposure to an environment with elevated levels of reactive oxygen species (as thiol groups on the cysteines were oxidized to form disulfide links) were responsible for making the tau proteins even more toxic to normal cell function.
The effect was overcome by the co-expression of antioxidants that helped natural processes clear away tau proteins and dramatically reduce the tau levels.
Thus identifying these chemical targets that trigger tau accumulation may lead to the formulation of new breakthrough treatments for Alzheimer’s disease and other tauopathies.
Source: Medindia