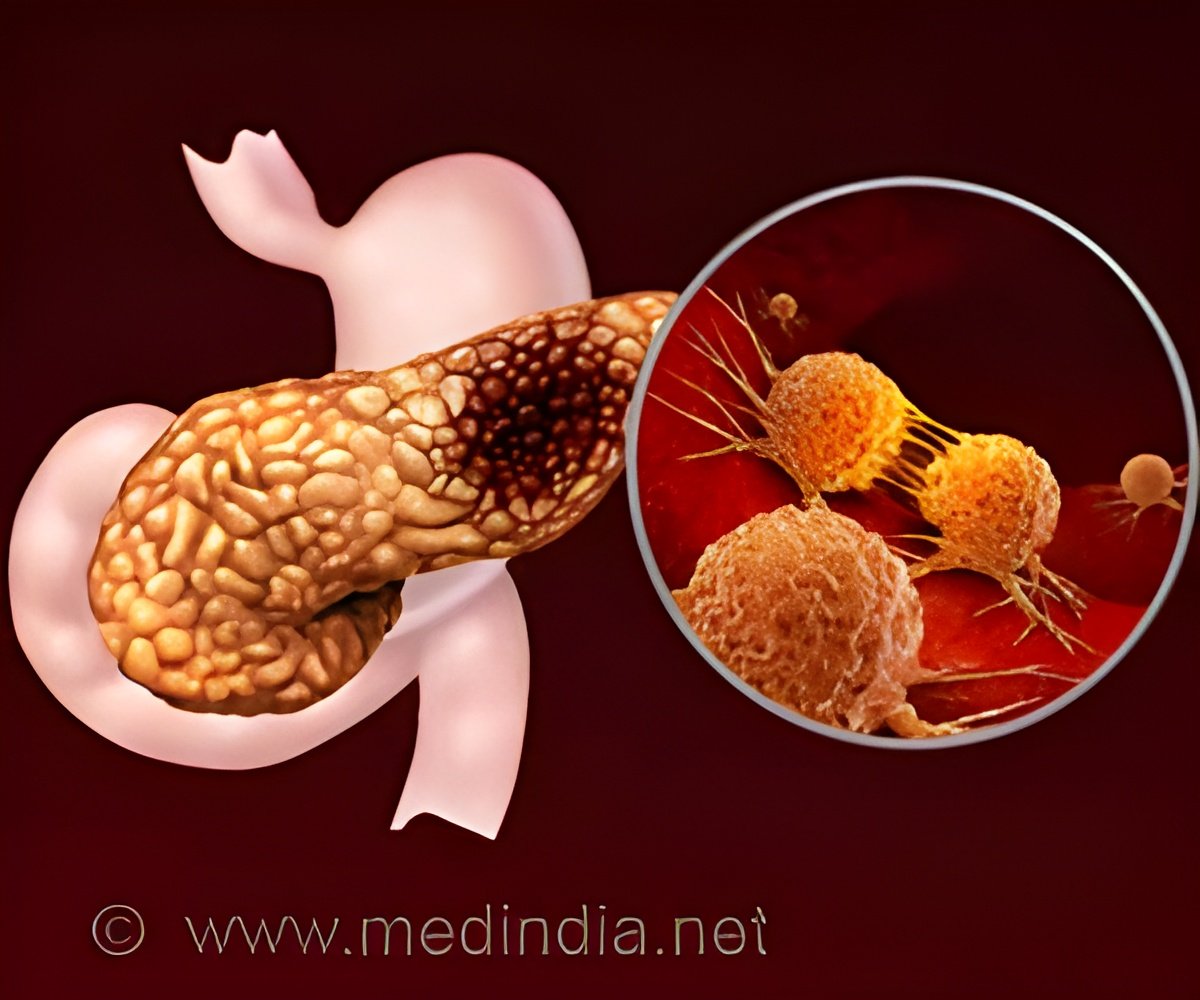
Pancreatic ductal adenocarcinoma is the most common type of pancreatic cancer, with a low five-year survival rate of 13%. It is the third largest cause of cancer death.
The microenvironment around the tumor presents a significant challenge. Dense tissue surrounding the tumor creates a barrier, inhibiting blood vessel growth and preventing immune infiltration.
According to Prabhani Atukorale, assistant professor of biomedical engineering at UMass Amherst, “Drug delivery is a tremendous challenge due to the architecture of the microenvironment of these difficult-to-treat tumors.” She also notes that this environment hinders the activation and infiltration of the body’s immune cells into the tumor(1✔ ✔Trusted Source
Nanoparticle delivery of innate immune agonists combined with senescence-inducing agents promotes T cell control of pancreatic cancer
).
Pancreatic cancer unfortunately does not respond to most conventional therapies, including chemotherapy and immunotherapy, which have revolutionized cancer therapy in the last decade.
Researchers demonstrated a new strategy for treating pancreatic cancer in mice models. The study, which was published in Science Translational Medicine, describes how a new nanoparticle drug-delivery system can activate an immune pathway when combined with tumor-targeting drugs.
Ruscetti’s previous research demonstrated that two cancer drugs (MEK inhibitor trametinib and CDK4/6 inhibitor palbociclib, or T/P) can promote blood vessel development, enabling greater T cell (and chemotherapy) delivery into the tumor.
Tricks in Pancreatic Cancer Treatment
However, the cancer “tricks” the immune system into thinking that the tumor is just a regular, healthy clump of cells. Since the T cells aren’t activated, simply having more of them present won’t clear the cancer.
Advertisement
Here’s where the researchers want to implement a trick of their own. The first pathway is called the stimulator of interferon genes (STING) pathway. STING recognizes viral infections in the body. “If we can trick the immune system into thinking that there is a viral-type infection, then we harness a very robust anti-tumor immune response to bring in for tumor immunotherapy,” Atukorale explains.
The researchers also wanted to activate the TRL4 pathway because it boosts the effects of STING activation. They use agonists, which are any chemicals that can trigger a biological response; in this case, in immune stimulatory pathways. However, getting these immunity-triggering chemicals through the tumor’s microenvironment is still a challenge.
Advertisement
Nanoparticle Drug Delivery in Pancreatic Cancer
The researchers’ solution: encapsulating the STING and TRL4 agonists in a novel design of lipid-based nanoparticles. The nanoparticles have several benefits. First, the research demonstrated that they are highly effective at delivering the agonists into the challenging tumor microenvironment.
The design also allows both of the agonists to be packaged together — a challenge since these two mix as well as oil and water. “It ensures that they are carried within the blood circulation together, they reach the same target cell together and are taken up together by the same target cell,” says Atukorale.
“We’re using biocompatible, lipid-based materials to encapsulate drugs that functionally work together, but don’t like to be next to each other, and then we can use engineering capabilities to build in various functionalities to direct them where they need to go,” she says.
The synergistic effect of the two agonists plus the T/P therapy proved effective: eight out of nine of the mice saw tumor necrosis and shrinkage. “And we had two mice that had complete responses, meaning the tumors completely went away, which is pretty striking,” says Ruscetti. “We’ve never seen that in this model before.”
There is still work to be done because the tumors returned after the mice were taken off of the treatment, but Ruscetti says it is still a very encouraging step toward a cure.
“If you go beyond pancreas cancer to other cancer types, you need a combination therapy to target the tumor and to target the immune system,” he adds. “This is a strategy to be able to do that.” Treatments for cancers like PDAC that could be derived from this study include mutations of colon cancer, lung cancer, liver cancer, and cholangiocarcinoma (cancer of the bile ducts).
Prabhani adds that the modular nature of this design allows for therapies that can be easily personalized for patients. “It’s sort of plug and play,” she says. “We can tailor the agonist ratios, the drug combinations, the targeting molecules, but keeping essentially the same platform. This is what will make it hopefully translational, but also tunable on a per-patient basis, because many of these cancer therapies need to be personalized.”
Finally, she nods to the power of collaboration between the two UMass institutions, saying, “This type of system is easily built when you have complementary, but multidisciplinary and cross-disciplinary, expertise.”
Reference:
- Nanoparticle delivery of innate immune agonists combined with senescence-inducing agents promotes T cell control of pancreatic cancer – (https:www.science.org/doi/10.1126/scitranslmed.adj9366
)
Source-Eurekalert