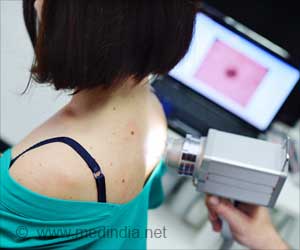
The test identifies a patient’s true risk of disease progression and provides anyone diagnosed with a non-ulcerated early-stage melanoma – accounting for around 75% of all new diagnoses – more accurate information about the risk of the disease spreading.
Now the scientists have demonstrated the mechanism in the skin which underpins the test, publishing the research in the British Journal of Dermatology.
Melanoma is increasing worldwide and every year more than 16,000 people in the UK and 96,000 people in the US are diagnosed with cancer.
In the new research, the authors explain how early-stage melanomas at risk of spreading secrete a growth factor, TGFβ2 which causes the reduction, or downregulation, of the proteins AMBRA1 and Loricrin – both of which are found in the skin overlying the tumor.
The growth factor TGFβ2 also causes the loss of claudin-1 leading to loss of the integrity of the skin and facilitating ulceration.
Senior author Professor Penny Lovat, Professor of Cellular Dermatology and Oncology at Newcastle University and Chief Scientific Officer at AMLo Biosciences explains: “Like mortar and bricks holding together a wall, AMBRA1, Loricrin and Claudin 1 are all proteins key to maintaining the integrity of the upper layer of the skin. When these proteins are lost gaps develop – like the mortar crumbling away in the wall. This allows the tumor to spread and ultimately ulcerate which we know is a process associated with higher risk tumors”.
A test, like AMBLor® which tells you that your tumor is genuinely low risk, helps significantly with the anxiety of an already very stressful situation.
Patients will understand what a low-risk result means. If the result is at risk, it completely justifies the significant number of interactions that you will have with the dermatology team over five years.
This test offers a personalized prognosis as it more accurately predicts if your skin cancer is unlikely to spread. This test will aid clinicians to identify genuinely low-risk patients diagnosed with early-stage melanoma and to reduce the number of follow up appointments for those identified as low risk, saving NHS time and money.
The British Skin Foundation is proud to support Prof Penny Lovat’s ground-breaking melanoma research. The development of the AMBLor test can alleviate stress and anxiety for patients caused by this potentially deadly skin cancer, whilst increasing efficiency and reducing costs to the NHS.
Currently, primary tumors are removed by surgery and pathologists study the biopsy under the microscope to determine the stage the skin cancer is at and the risk of it spreading (metastasis).
Even if defined as low risk, the patient is followed up in the clinic for as long as five years – and it is these patients that the test can identify.
The AMBRA1 and loricrin test is accredited by UKAS and is already available through a private referral service from the spin-out company, AMBLo Biosciences.
Source: Medindia