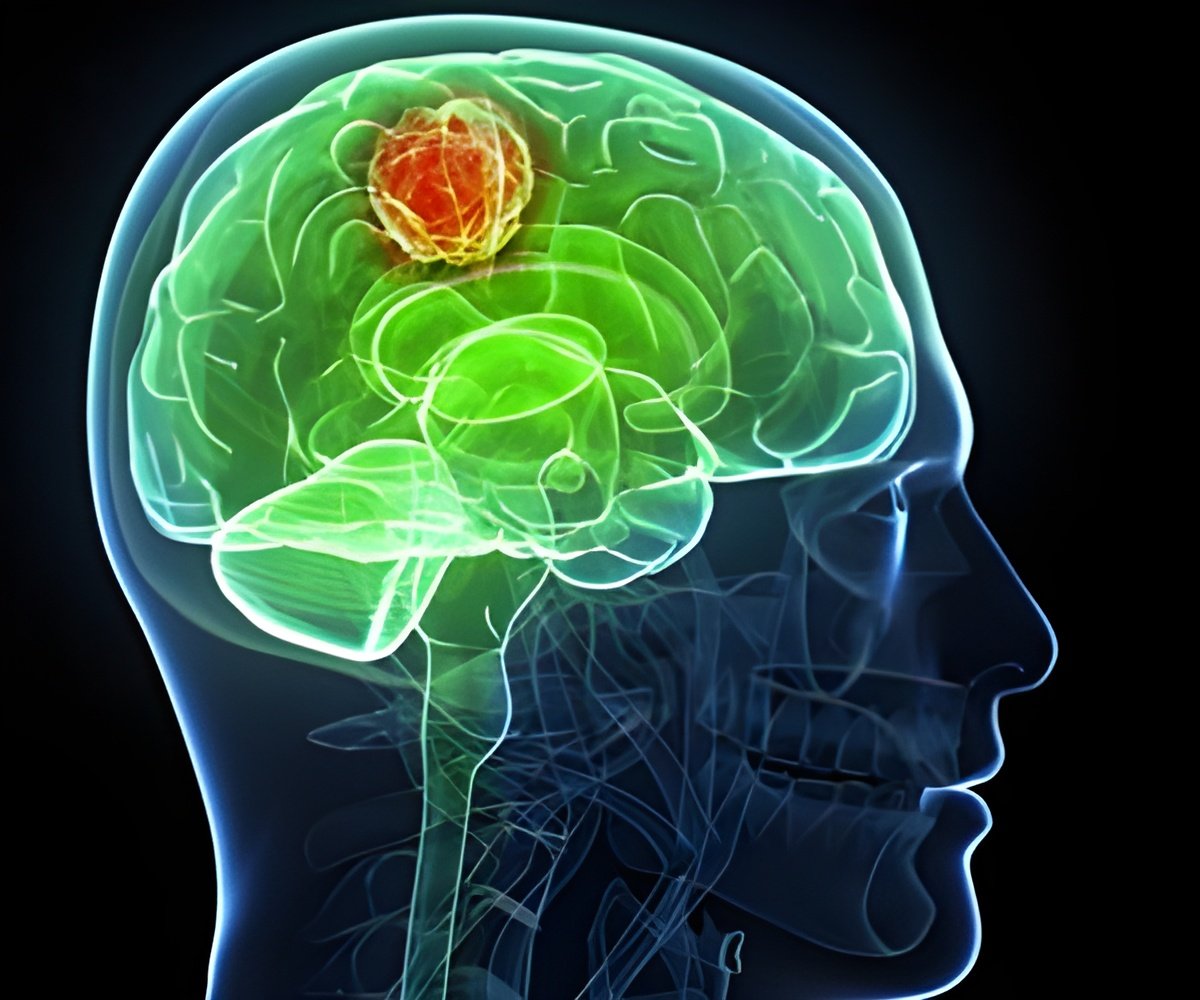
An innovative method for treating the most aggressive form of brain cancer, glioblastoma, has demonstrated significant potential in pre-clinical studies. The study results are optimistic about the possibility of extending the current average survival rate of 18 months (1✔ ✔Trusted Source
Targeted Alpha Therapy for Glioblastoma: Review on In Vitro, In Vivo and Clinical Trials
).
Targeted alpha therapy (TAT) is being recognized as a promising supplementary approach for treating glioblastoma (GB), a challenging disease that has puzzled oncologists for many years due to its aggressive characteristics and high resistance to current treatments.
The survival rate of GB patients is less than 5-10% at five years, despite the current standard treatment for GB being surgery, followed by external beam radiotherapy and the chemotherapy drug, temozolomide. This has driven the scientists to explore alternate options.
Advertisement
Viability of Targeted Alpha Therapy (TAT) in Glioblastoma
Scientists at the University of South Australia are currently carrying out independent experiments involving TAT and have examined previous clinical research to evaluate the potential of targeted alpha therapy as a viable treatment for recurrent glioblastoma.
The evidence supporting TAT is outlined in a recent paper published in Targeted Oncology by UniSA Ph.D candidate Maram El Sabri, medical radiation physicist Professor Eva Bezak, and oncologist Professor Frank Saran.
“Unlike external beam radiotherapy, which delivers radiation more diffusely over a larger volume, TAT delivers high amounts of lethal radiation to the tumour at very short range, hitting its target without significantly affecting surrounding healthy tissue,” says Maram.
“Alpha particles are up to 10 times more potent, when compared to standard photon radiation therapy, killing the cancer cells or at the very least slowing their future growth by damaging their DNA.”
Advertisement
Challenges of Glioblastomas: TAT Overcomes Them!
Glioblastomas pose a challenge due to their rapid growth and tendency to infiltrate surrounding healthy brain tissue, complicating the administration of precise radiation therapy doses by oncologists.
Studies on animals have shown that a limited number of targeting agents are able to successfully penetrate the blood-brain barrier (BBB) to reach the cancerous tissue, but those that manage to do so often lead to adverse effects in the surrounding healthy tissue.
During pre-clinical trials, TAT has demonstrated a 16.1% improvement in survival rates for individuals with newly diagnosed glioblastoma, and a 36.4% increase for those with recurrent tumors. In addition, research indicates that the treatment has minimal negative impacts on patients.
Professor Bezak, a co-author, mentions that TAT was initially suggested for cancer treatment over two decades ago by the renowned Australian research scientist, Professor Barry Allen, who passed away in 2019 due to cancer.
“He was ahead of his time. It has taken this long for TAT to be gradually accepted by clinicians and for animal (pre-clinical) and human (clinical) studies to be undertaken,” Prof Bezak says.
“Pre-clinical studies show very promising results. Alpha emitters are up to 10 times more toxic to cells than gamma radiation, which are used in external beam radiation. Also, compared to the cost of current immunotherapy or molecular targeting drugs, targeted alpha therapy is relatively cheap.”
Professor Frank Saran, an Adjunct Clinical Professor at UniSA and experienced radiation oncologist, remarked minimal advancements in the treatment of glioblastoma have been made over the past few decades, leading to a resurgence in interest in TAT.
“The most exciting development was the discovery of the chemotherapy drug temozolomide in the 1980s but that has only improved the expected median survival by around three months,” he says.
Advertisement
Why Research on Glioblastoma Has Been Limited?
“Research into this area is very low for several reasons. First, glioblastoma is a rare cancer so does not affect large swathes of the population. It also has extremely low survival rates and there is a long-standing history of failed studies in this area. Unfortunately, pharmaceutical companies are often not willing to invest money in GB because it has a low probability of success and is not commercially viable.”
Maram, in her Ph.D programme, is creating a computational model to determine the most efficient method of delivering TAT to the brain, post-surgery. This model will also be used in conjunction with traditional radiation therapy and chemotherapy to specifically target any remaining cancer cells and enhance the effectiveness of radiation on the tumor.
“I am excited to find out if we can find the right dosing and radiation range by adding TAT to the conventional treatment options. If this is successful, we might see some significant results in terms of extending a patient’s life,” she says.
In conclusion, Glioblastomas are difficult to treat because of their fast growth and ability to spread beyond visible tumors into healthy brain tissue. New therapies like TAT offer hope for improving survival rates and quality of life of patients.
Reference:
- Targeted Alpha Therapy for Glioblastoma: Review on In Vitro, In Vivo and Clinical Trials – (https://link.springer.com/article/10.1007/s11523-024-01071-y)
Source-Medindia