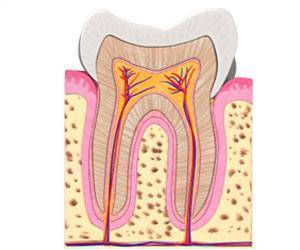
Tooth enamel or the outer layer of the tooth gives protection from the mechanical stresses incurred by chewing and helps them resist decay. Enamel is the hardest tissue in the human body and is formed by specialized cells called ameloblasts (teeth-forming cells).
When tooth formation is complete, these cells die off. Consequently, the body has no way to repair or regenerate damaged enamel, and teeth can become prone to fractures or be subject to loss. To create ameloblasts in the laboratory, researchers first had to understand the genetic program that drives fetal stem cells to develop into these highly specialized enamel-producing cells.
Exploring the Role of Stem Cells in Tooth Repair
To do this they used a technique called single-cell combinatorial indexing RNA sequencing (sci-RNA-seq), which reveals which genes are active at different stages of a cell’s development.
Advertisement
This is possible because RNA molecules called messenger RNA (mRNA), carry the instructions for proteins encoded in the DNA of activated genes to the molecular machines that assemble proteins. That is why changes in the levels of mRNA at different stages of a cell’s development reveal which genes are turned on and off at each stage().
By performing sci-RNA-seq on cells at different stages of human tooth development, the researchers were able to obtain a series of snapshots of gene activation at each stage. They then used a sophisticated computer program, called Monocle, to construct the likely trajectory of gene activities that occur as undifferentiated stem cells develop into fully differentiated ameloblast.
The computer program predicts how you get from here to there, the roadmap, the blueprint needed to build ameloblasts. With this trajectory mapped out, they were able to coax undifferentiated human stem cells into becoming ameloblasts.
They did this by exposing the stem cells to chemical signals that were known to activate different genes in a sequence that mimicked the path revealed by the sci-RNA-seq data. In some cases, they used known chemical signals. In other cases, collaborators from the UW Medicine Institute for Protein Design created computer-designed proteins that had enhanced effects.
They also identified for the first time another cell type called a sub-odontoblast, which they believe is a progenitor of odontoblasts, a cell type crucial for tooth formation.
Together these cell types could be induced to form small, three-dimensional, multicellular mini-organs, called organoids. These organized themselves into structures similar to those seen in developing human teeth and secreted three essential enamel proteins: ameloblastin, amelogenin, and enamelin().
These proteins would then form a matrix. A mineralization process that is essential for forming enamel with the requisite hardness would follow.
The research team now hopes to refine the process to make an enamel comparable in durability to that found in natural teeth and develop ways to use this enamel to restore damaged teeth. This kind of approach that would create enamel in the laboratory could be used to fill cavities and other defects in the future.
References:
- Baena, A.R., Casasco, A. & Monti, M. Hypes and Hopes of Stem Cell Therapies in Dentistry: a Review. Stem Cell Rev and Rep 18, 1294-1308 (2022).( https://link.springer.com/article/10.1007/s12015-021-10326-4)
- Hemeryck, Lara et al. Organoids from human teeth show epithelial stemness phenotype and differentiation potential. Cellular and molecular life sciences: CMLS vol. 79,3 153. 26 Feb. 2022.(https://link.springer.com/article/10.1007/s00018-022-04183-8)
Source: Eurekalert